Where Others Stop, We Begin



“My team and I strongly believe that regulatory approval should never be the ultimate goal. Pharmaceuticals and medical devices must consistently demonstrate meaningful clinical value.”
Dr. Kai Ulf Markus
“My team and I strongly believe that regulatory approval should never be the ultimate goal. Pharmaceuticals and medical devices must consistently demonstrate meaningful clinical value.”
Dr. Kai Ulf Markus


Why Work With Us
In my opinion, anyone conducting a clinical trial should at the very least…
- have experience in direct patient care.
- have experience in diagnosing and treating patients as a physician.
- possess scientific expertise and have authored peer-reviewed publications.
- have been involved in the development and manufacturing of medical devices, IVDs, or pharmaceuticals—both in small-scale and large-scale production environments.
- Arzneimittel– / Medizinprodukte und IVD– Produkte weltweit zugelassen haben.
- Arzneimittel– / Medizinprodukte und IVD– Produkte weltweit vermarktet haben.
- have planned and overseen clinical studies themselves.Eigene Klinische Studien geplant und betreut haben.
just to be even remotely qualified to support our clients.
I’ll admit—my expectations are high.
Our team combines the expertise of medical doctors, engineers, and biotechnologists
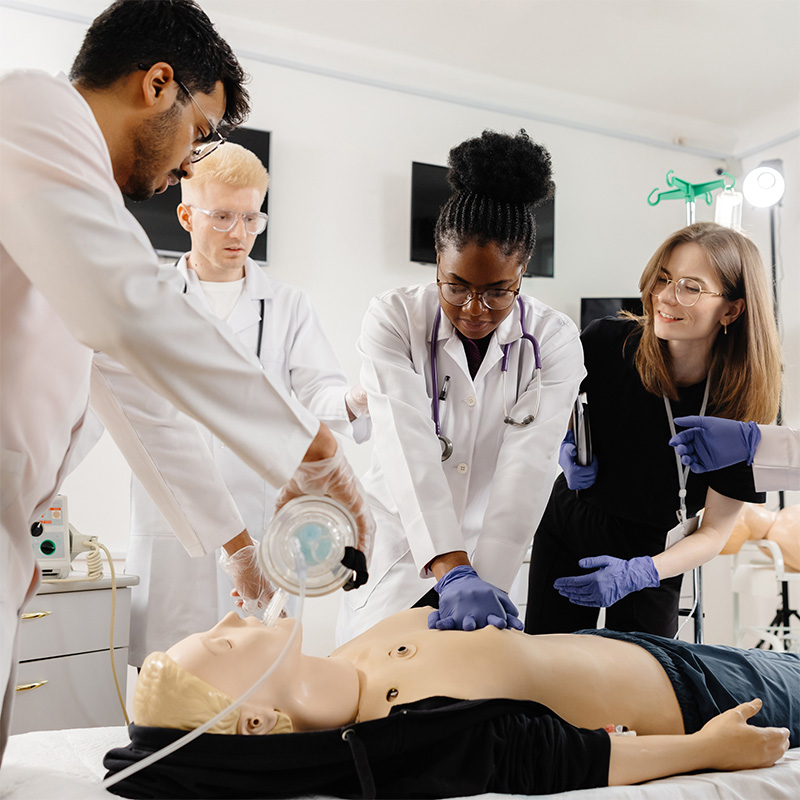
Clinical Research
Why a CRO? Why Outsource?
Contract Research Organisations (CROs) are essential partners in the development of new pharmaceuticals, medical devices, and diagnostics. They support industry efforts to test, refine, and bring innovations to market—faster and more effectively.
Key benefits of outsourcing:
Deep expertise in regulatory affairs, pricing and reimbursement, competitive analysis, clinical research, and commercial strategy.
We guarantee access to state-of-the-art technologies and the most advanced systems for data management, product development, research analytics, and other clinical research services.

Significant cost reductions through accelerated timelines and reduced internal resource requirements.

This eliminates the need for the sponsor to maintain their own personnel, infrastructure, laboratories, or office space for the execution of clinical studies.
Access to a global network of research sites, monitors, Clinical Research Associates (CRAs), physicians, and Key Opinion Leaders (KOLs).
We work with a wide range of sponsors, which gives us a breadth of experience that goes far beyond what a single large pharmaceutical or medtech company can typically offer.

At VYSYO, we adhere to clinical compliance standards on a daily basis—requiring in-depth internal expertise in regulatory requirements and audit readiness, including Good Clinical Practice (GCP) and Good Laboratory Practice (GLP) audits.

Our standardised approach ensures high quality—at a significantly reduced cost.
What Our Clients Say
CEO of a SME in Digital Health





CEO of a SME in Digital Health





“Pragmatic, competent, goal-oriented – we got the support we needed from Dr. Kai Markus. The service was provided in an efficient manner. We have highly valued Dr. Markus’s flexibility, availability and pleasant manner throughout our collaboration. As a result we were able to successfully initiate a complex clinical / regulatory project new to our company.
I can only say a heartfelt thank you for the cooperation and the satisfactory result.”
Head of Research & New Technology





